Gene & Cell Therapies – Q&A
Increasingly, people hear about transformative or curative Gene & Cell therapies. These state-of-the-art techniques certainly can revolutionise medicine as we know it. Bringing high hope for millions of patients in Europe as they become available in some countries, they also spark a lot of questions, starting with what these innovative therapies are all about.
With this page, EUCOPE aims to answer most of the frequently asked questions around gene and cell therapies. If you have any questions, please feel free to contact us.
Happy reading!
- What is a gene therapy?
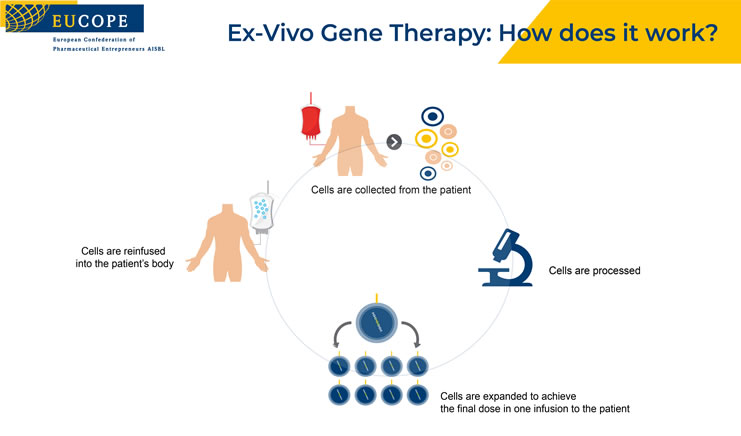
Gene therapy is the use of genetic material to treat genetic diseases. This may involve adding a wild type copy of the gene (gene addition) or altering a gene with mutation to the wild type gene (gene editing). The treatment may take place outside of the body (ex vivo) or inside the body (in vivo).[1]
Gene therapy medicines are a type of advanced therapy medicinal products (ATMPs). They contain genes that lead to a therapeutic, prophylactic or diagnostic effect. They work by inserting ‘recombinant’ genes into the body, usually to treat a variety of diseases, including genetic disorders, cancer or long-term diseases. A recombinant gene is a stretch of DNA that is created in the laboratory, bringing together DNA from different sources.[2]
[1] European Society of Gene & Cell Therapy, https://www.esgct.eu/Useful-Information/Gene-and-cell-therapy-glossary.aspx, accessed 07 March 2019.
[2] European Medicines Agency, https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview, accessed 07 March 2019.
- What is a cell therapy?
Cell therapy is the transfer of intact, live cells into a patient to help lessen or cure a disease. The cells may originate from the patient (autologous cells) or a donor (allogeneic cells). The cells used in cell therapy can be classified by their potential to transform into different cell types. Pluripotent cells can transform into any cell type in the body and multipotent cells can transform into other cell types, but their repertoire is more limited than that of pluripotent cells.[1]
[1] American Society of Gene & Cell Therapy, https://www.asgct.org/education/more-resources/gene-and-cell-therapy-faqs, accessed 07 March 2019.
- What is the difference between gene and cell therapies?
Gene therapy involves the transfer of genetic material, usually in a carrier or vector, and the uptake of the gene into the appropriate cells of the body.
Cell therapy involves the transfer of cells with the relevant function into the patient.[1]
[1] American Society of Gene & Celle Therapy, https://www.asgct.org/education/more-resources/gene-and-cell-therapy-faqs, accessed 07 March 2019.
- In which therapeutic areas can gene and cell therapies be used?
Gene therapies and cell therapies have the potential for high therapeutic gain for a broad range of diseases, improving the quality of life of persons living with severe or chronic diseases.
They offer a promising alternative or adjunct treatment for symptoms of many diseases, such as cancer, rheumatoid arthritis, diabetes, Parkinson’s disease, Alzheimer’s disease, hemophilia, and neurodegenerative diseases.
In oncology, gene therapies focus on eliminating the cancer cells, blocking tumor vascularization and boosting the immune response to tumor antigens.[1]
[1] American Society of Gene & Celle Therapy, https://www.asgct.org/education/more-resources/gene-and-cell-therapy-faqs, accessed 07 March 2019.
- What is the regulatory/legislative framework in Europe?
Gene and cell therapies are classified by the European Medicines Agency (EMA) as Advanced Therapy Medicinal Products (ATMPs), which are medicines for human use that are based on gene, tissues or cells. ATMPs is a large category also comprising tissue-engineered medicines, in addition to gene and cell therapies.
The Regulatory framework for gene and cell therapies in the EU offers a stable and solid environment.
All advanced therapy medicines are authorised centrally via the European Medicines Agency (EMA). They benefit from a single evaluation and authorisation procedure.
The overall legislative framework for gene and cell therapies is provided by the Regulation 1394/2007 on Advanced Therapy Medicinal Products, amending Directive 2001/83 and Regulation 726/2004.
In addition, the European Directive 2009/120/EC (amending Directive 2001/83/EC) updated the definitions and detailed scientific and technical requirements for gene therapy medicinal products and somatic cell therapy medicinal products.
If an ATMP is also an Orphan Medicinal Product (OMP), then the EU Regulation 141/2000 on OMPs applies.
[1] https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapies/legal-framework-advanced-therapies
- Are gene and cell therapies available in Europe?
As of February 2020, they were 10 ATMPs approved by the European Medicines Agency, providing solutions to various conditions such as corneal diseases, pneumococcal infections, lymphoma, melanoma, cartilage diseases, immunodeficiency, Graft vs Host Disease, fistulae.[1]
[1] EMA, list of approved ATMPs, as of 07 March 2019.
- What is a CAR-T cell therapy?
CAR-T cell therapy is a type of treatment in which a patient’s T cells (a type of immune system cell) are changed in the laboratory so they will attack cancer cells. T cells are taken from a patient’s blood. Then the gene for a special receptor that binds to a certain protein on the patient’s cancer cells is added in the laboratory. The special receptor is called a chimeric antigen receptor (CAR). Large numbers of the CAR T cells are grown in the laboratory and given to the patient by infusion.[1]
[1] National Cancer Institute, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy, accessed 07 March 2019.
- What are the benefits/risks of gene and cell therapies?
Gene and cell therapies bear with them the exciting promise of potentially alleviating the cause of genetic and acquired diseases. While other treatments manage the symptoms of a disease, gene and cell therapies can be one-time disease modifying treatments that can transform and save lives.
Risk is an inescapable effect of any medical treatment. Gene and cell therapies are complex and sophisticated procedures and are therefore no exception to the rule.
A gene can only be inserted into a cell through a carrier, called a vector (most often a virus). This technique presents some risks such as an unwanted immune response or an infection caused by the vector.[1]
As with all therapies, patients are strongly encouraged to seek advice from their practitioners and clinicians.
[1] https://www.mayoclinic.org/tests-procedures/gene-therapy/about/pac-20384619
- What are the challenges associated with the delivery of gene and cell therapies?
Gene and cell therapies can be seen as revolutionary treatments, potentially transforming the way patients are treated and, hopefully, cured. These new therapies require adequate and agile health systems to adjust and provide fair assessment, leading to an equal access for patients.
Evidential Uncertainty
A first challenge lies within the evidential uncertainty associated with gene and cell therapies. The current health technology assessment (HTA) models are not fit-for-purpose. A solution could be to have more flexible HTA processes which are tailored for such therapies, recognizing the limited number of patients and the absence of comparators, and taking into account the highly positive short term and best available scientific evidence. Those measures can be strengthened by the alignment of registries requirements across Member States, facilitating data collection to demonstrate ongoing safety and efficacy and improve evidence base.
Gene and cell therapies typically involve costly research, development, and manufacturing costs and are associated with greater logistical demands for patients. Another factor to consider is that gene and cell therapies are designed to be curative, and so the cost of therapy can be weighed against that of lifetime treatment.
Affordability
It is necessary to consider the affordability issues for health systems, in a multi-year longer-term deal structure. Gene and cell therapies have the potential to be transformative, by bringing benefits to the patients, but also ultimately savings on medicines budget on the long term, as well as a reduction/optimization of caregiver time. A collaborative approach to funding/reimbursement/payment mechanisms can ensure appropriate pricing systems, with adequate incentives for research and innovation are in place.
Availability
Gene and cell therapies are highly personalised treatments with complex manufacturing and distribution processes. Many of these therapies will be available in only a small number of treatment centres in Europe with the critical expertise in manufacturing and the administration of therapy including essential safety monitoring after infusion of the cell and gene therapies. Even patients in larger countries may have to travel to a different region or country to receive these treatments.
A proper application of the Directive on Cross-border healthcare (2011/24/EU) can help ensuring no patient is barred from accessing those revolutionary treatments.
Research
European university hospitals have been the birthplace of some of the technologies which had driven advancements in gene and cell therapies. This should be acknowledged and give enough reasons to continue supporting research and science at the EU level. However, the current clinical trials infrastructure extremely varies across Europe, putting at risk the further development of research in advanced therapies.
- Can gene and cell therapies be used to prevent the onset of diseases?
Gene therapies have the potential to prevent certain diseases, by turning off the mutated genes causing the disease, or by turning on healthy genes to inhibit the disease. Early research findings indicate gene therapies could indeed prevent the onset of diseases, most notably in brain-related diseases. However, further results are expected before any applications for patients could be envisaged.
- What can be done now?
With the first gene and cell therapies a few months away from entering the EU market, it is highly relevant for stakeholders to get together and find solutions to allow European patients to gain quick access to these revolutionary therapies.
EUCOPE and its members work hand in hand in raising awareness. of these new therapies and provide a trusted platform to elaborate solutions in terms of access, payment models and delivery challenges. In that regard, see our Position Paper on Gene & Cell Therapies.
Together with other stakeholders, EUCOPE takes part various initiatives to deliver better information and contribute to improve patient access to gene and cell therapies. The RARE IMPACT is a stellar example, bringing together patient representatives, regulators, HTA bodies, payers, clinicians and industry in a patient- focused collaboration to assess the challenges in patient access to gene and cell therapies along with proposing actionable solutions.