Our Partnerships
Fostering a cycle of innovation for rare disease patients in Europe
We believe that in order to improve the entire health innovation environment in Europe, policy solutions need to be devised all along the lifecycle of medicines, from very early research to patient access.
EUCOPE’s membership consists of European and global companies committed to ensuring that Europe remains an attractive location to undertake research and launch products for people living with rare diseases.
Our Commitment is to lead and engage in partnerships spanning the entire medicine lifecycle to find actionable solutions that benefit our members, the patients they serve and healthcare systems overall.
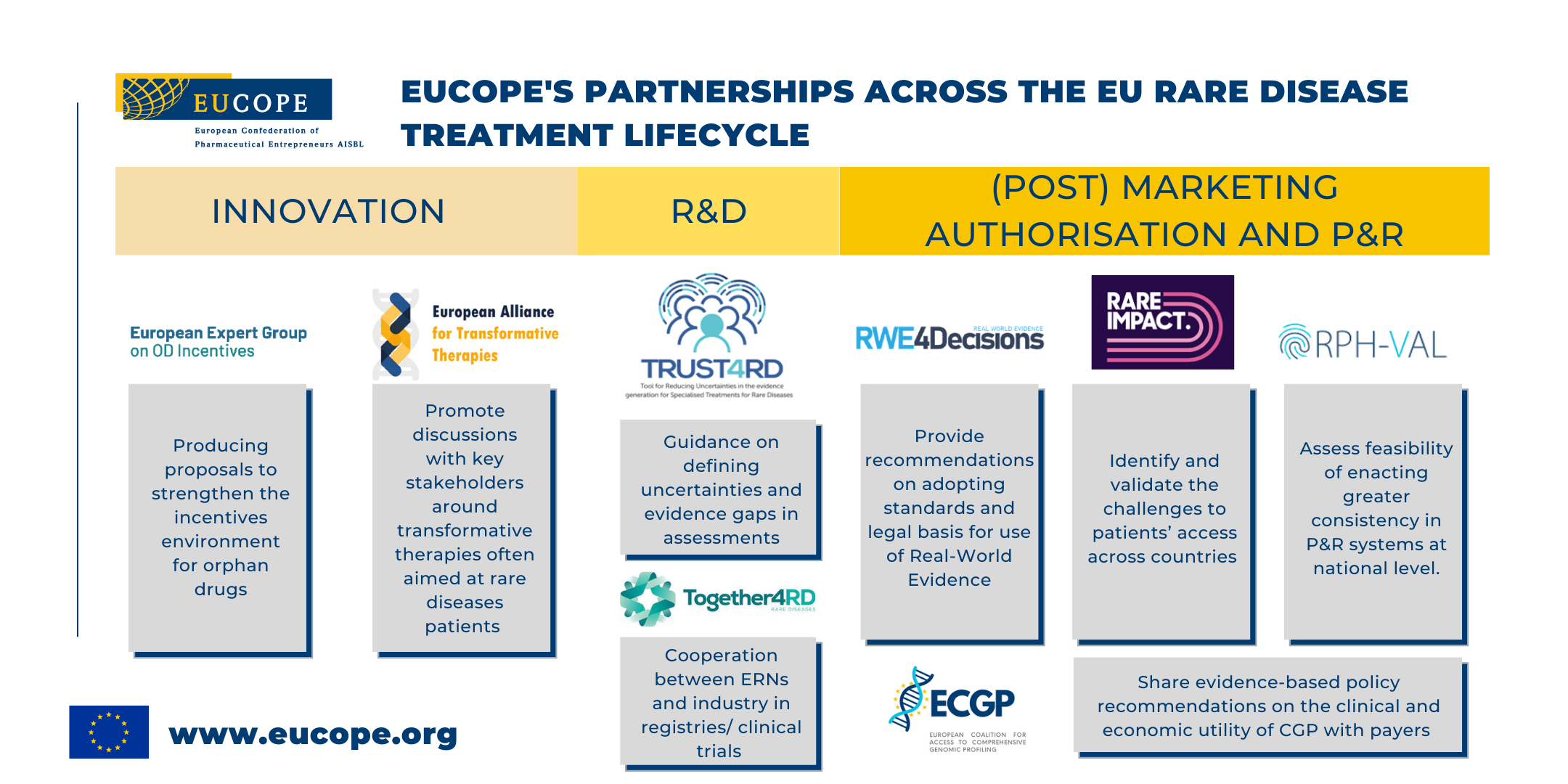
The following are some of our current partnerships:

The European Expert Group on Orphan Drug Incentives is multi-stakeholder initiative producing proposals to strengthen the incentives environment for orphan drugs.

The European Alliance for Transformative Therapies (TRANSFORM) is a multi-stakeholder Alliance that connects Members of the European Parliament (MEPs) and policy-makers with patient groups, medical experts and associations, scientists, researchers, industry actors, networks and other relevant stakeholders. TRANSFORM aims to foster effective dialogue and provide evidence-based policy recommendations to enable safe and timely patient access to cell and gene therapies, whilst ensuring the sustainability of healthcare systems.

Together4RD aims to identify and launch areas to pilot cooperation between ERNs and industry in registries and clinical trials (leveraging EUCOPE advisory roles in ERICA and EJPRD)

RWE4Decisions is a payer-led multi-stakeholder initiative aimed at developing models to include RWE in HTA assessment. The group developed rrecommendations on adopting standards and legal basis for use of Real-World Evidence

Rare Impact is a Patient-focused collaboration that seeks to improve patients’ access to Gene and cell therapies. The initiative aims to identify and validate the challenges to patients’ access across countries

ORPH-VAL, the group of experts developed a set of principles to be applied across European markets to assess feasibility of enacting greater consistency in P&R systems assessment of OMPs at national level. Building on existing cooperation mechanisms ( e.g. MoCA-OMP)

The European Coalition for Access to Comprehensive Genomic Profiling (ECGP) is a multi-stakeholder initiative to improve cancer care through increased routine clinical access and reimbursement of Comprehensive Genomic Profiling (CGP). The ECGP identifies and shares best practices, and develop evidence-based policy recommendations on the clinical and economic utility of CGP for payers and other decision-makers, based on multi-stakeholder and multi-disciplinary perspectives.

The Rare Disease Moonshot aims to boost public-private partnerships in the field of rare diseases that have few or no treatment options and research activities. The Moonshot Collective is a network of stakeholders from the translational research and development ecosystem who work together to build trust, share expertise, reduce research fragmentation and foster greater collaboration among organisations.

Partnerships in Focus series with Takeda’s Toon Digneffe

Partnerships in Focus series with Astra Zeneca/Alexion’s Maciej Gajeweski


